All materials implanted into the human body, whether synthetic or natural, are instantly barraged with thousands of surface-active proteins, saccharides, lipids, and smaller molecule solutes found in all physiological fluids: multiple interfacial forces are involved in this adsorption, and several thousand known serum proteins participate in this response.
Cells, as larger, slower diffusing species, arrive on an implant surface at later times, when smaller molecule adsorption processes are well-advanced kinetically and thermodynamically.
In short, control of this biological adsorption response to biomaterials has proven extremely challenging. To date, all biomaterials developed or implanted react with physiological fluids to greater or lesser extents; none is “inert”, none fully rejects this biological adsorption in vivo.
Therefore, the biocompatible properties of all implanted biomaterials, however defined, are currently inseparable from ubiquitous adsorption of this interfacial protein film that directly apposes the host tissue. The surface competition eventually produces an adherent conditioning film of surface-adsorbed species of mixed composition and heterogeneity on all biomaterials.
Biomaterials surface chemistry influences the kinetics and thermodynamics of this response, but often is observed empirically to exhibit a disappointing result in vivo: biomaterials integration with host cells and tissues, and desired biocompatibility performance requirements (i.e., healing) are often sub-optimal in many tissue compartments and surgical applications.
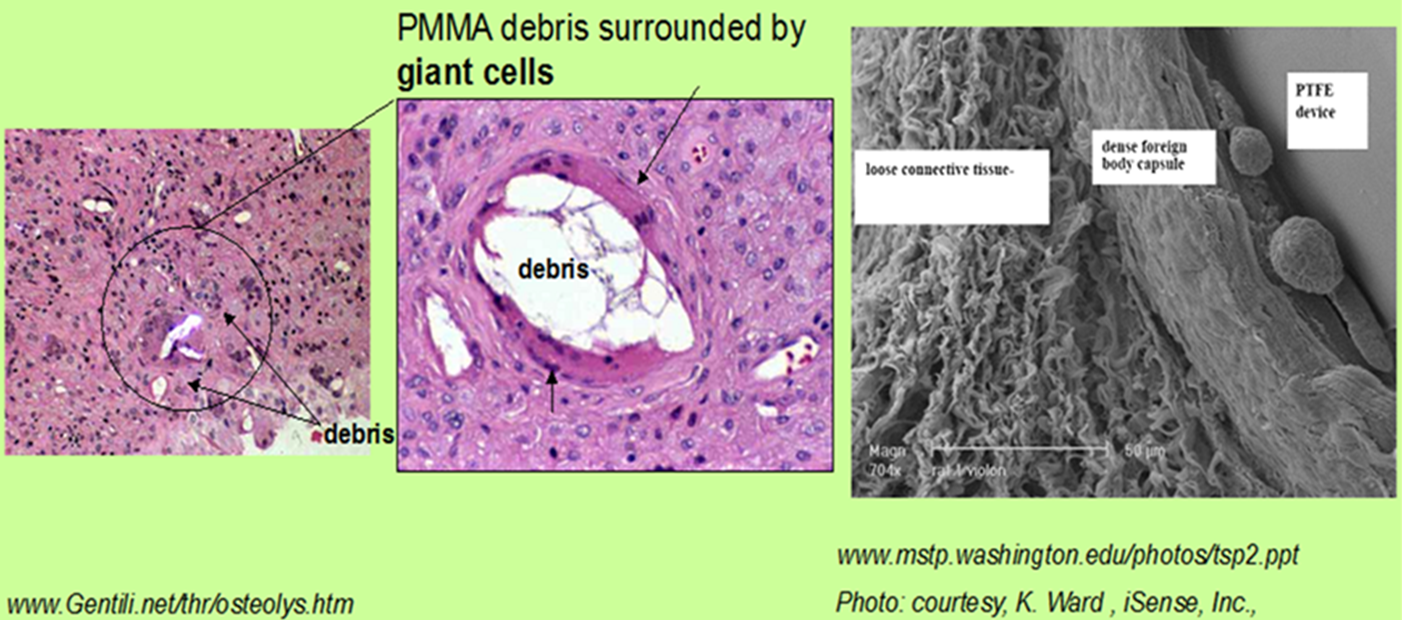
This leads to the ubiquitous host-implant “foreign body response”. Implanted surfaces cannot yet be reliably endowed with one of two frequently desired bio-performance endpoints sought to improve host response and device integration: (1) biased, non-commensurate, selective uptake of extracellular matrix proteins often found in trace quantities in serum to surfaces in order to promote reliable long-term cell adhesion, or (2) complete repulsion of all protein adsorption to render the surface non-biologically reactive and perhaps non-inflammatory.
An additional strategy now uses local release of anti-inflammatory or growth-promoting drugs from implant surfaces to condition the local implant tissue bed with appropriate pharmacology to mitigate adverse host-implant responses.
The Grainger group has produced several types of basic macrophage biology and drug-releasing device-based research approaches to study the foreign body response in animal implant models.

